drug dilution guidelines
Pharmacy Practice and Development Division. Benchmade - diluents should not be used even if sterilized as these may contain pyrogens that cannot be removed by filtration or heating.
6242019 11 Weight based medication doses measured minute Formula mlhr body weight x dose x 60 min.

. Prepare a solution at 50 mcgmL Take 1 mL of 250 mcg mL solution and mix with 4 mL NS to obtain a final concentration of 50 mcgmL or 250 mcg 5 mL. Concentration of drug Concentration of Drug mg x 1000 volume of the solution If you are diluting in 50 ml. As suggested by the variability of dilution methods and volumes less than half of respondents 43 reported organizational policies or guidelines on dilution.
Water loading works by increasing the excess water in your body so the bladder will refill with excess water before the normal mixture of byproducts and the normal level of water used to expel these byproducts accumulates back. Moreover those pharmaceutical products requiring dilution or reconstitution prior to use also require in-use stability studies. Intravenous admixtures preparation and infusion guidelines This reference contains standard dilutions including IV admixture drug concentration infusion volumes and infusion rates.
STEP-6 PEDI OR SETUP - AIRWAY SETUP. Answer We need to dilute 250mL of a 25 wv solution to 625mL to produce a 10 wv solution. How Water Dilution for Drug Testing Works.
Dilution should never be done with a manufactured prefilled saline flush syringe. Pharmaceutical products delivered in multidose containers require in-use stability studies. Ministry of Health Malaysia.
Please consult specialized references for validation of pediatric doses. Most of the remaining. STEP-8 PEDI OR SETUP - IV TRAY AND LINE.
Dose of 100 mcg and more. All drugs requiring dilution must be diluted and maintained using sterile technique. This guideline was prepared to facilitate the healthcare staffs in preparing the dilutions of drugs which are available in the Ministry of Health MOH drug lists.
Antimicrobial Dilution Protocol. Dilution samples DS expire on the 90th day following dilution from original stock containers. Pediatric OR Setup HOME.
Combining mixing or altering ingredients of a pharmaceutical grade drug to create a medication tailored to needs of an individual. If the dosage calculation does not require wt remove wt from the formula. Mlhr body weight x dose x 3 mg.
STEP-2 PEDI OR SETUP - BREATHING CIRCUIT. Each monograph contains stability data administration guidelines and methods of preparation. Drug dilution 1.
STEP-3 PEDI OR SETUP - SUCTION. Institutions should provide the appropriate diluent along with the drug to ensure safety. A dilution refers to process of adding additional solvent to a solution to decrease the concentration.
May rarely cause allergic reactions. In-use stability can be described as how well a pharmaceutical product remains stable during in-use within a particular closure system. Use the correct diluent.
Reperfusion arrhythmias increased risk of bleeding. Drug Dilution and Storage Guidelines Most of the drugs used for laboratory animals ie mice rats need to be diluted for accurate dosing. Heparin GTN dobutamine dopamine.
Adrenaline infusion Preparation 1 amp 1ml 11000 1mgml Dilute 30mg 3mls adrenalin with 47mls of NS in 50mls syringe. IV slow push over at least 5 minutes Compatible with D5W NS. To avoid waste and contamination potential only make up what is needed.
The availability of this guideline will assist in the expansion of quality clinical care pharmacy services throughout MOH facilities. No respondents described a dilution process that would result in a specific concentration eg add 4 mL of diluent to 1 mL of drug to equal xx mg5 mL. The information provided in this guideline mainly extracted from the package insert for the brand of injectable drugs used and valid at the time.
Where no in divided doses are indicated in the maximum daily dose maximum dose may be given in. Dilution of IV push medications should be done only when either required by the manufacturer based on peer-reviewed evidence or by institutional guidelines. Manufacturers recommend do not mix with any other drugs Reconstituted vial stable for 24 hours in the fridge or 8 hours at room temperature.
DRUG DILUTION TABLE GENERAL GUIDELINES FOR DRUG PREPARATION AND SAMPLE COMPUTATION 𝒙 700 mg Piperacillin Note. STEP-4- PEDI OR SETUP - OR TABLE AND PROPS. STEP-7 PEDI OR SETUP - MEDICATION DESKTOP.
No dilution required 250 mcgmL Dose of 999 mcg and less. A Abciximab reopro Acetaminophen Acetazolamide Diamox. Maximum Daily Doses are indicated for ADULT PATIENTS only.
Section 3 Concentrated Waters Concentrated waters such as rose water peppermint water and chloroform water are used to produce single-strength solutions. Dilution Guideline for Injectable Drugs serves as a general reference for the healthcare professionals in the Ministry of Health on how to prepare dilution of certain injectable drugs before administering to the patient. Intermittent infusion 50mg over 30minutes and then 35mg over.
Always follow UAB ARP veterinary recommendations for dilution concentration and dosages in animals. However this procedure may also be considered compounding. STEP-5- PEDI OR SETUP - MONITORS.
Sterile needles and syringes must be used to transfer drugs and diluents into sterile empty vials see image below. The average healthy adult has a bladder that will comfortably hold 16 ounces or 2 cups of liquid. Compounding is defined as.
Diluents should be sterile pharmaceutical preparations such as normal saline for injection bacteriostatic saline for injection or lactated Ringers solution for injection. Use sterile saline or water as the diluent.

Photo 46 Medication Dilution Chart Posted At The Nursing Station For Download Scientific Diagram
Some Iv Medications Are Diluted Unnecessarily In Patient Care Areas Creating Undue Risk Institute For Safe Medication Practices

Serial Vs Direct Dilution Time To Apply New Thinking To Ic50 Determination And Dose Response Analysis Drug Discovery World Ddw
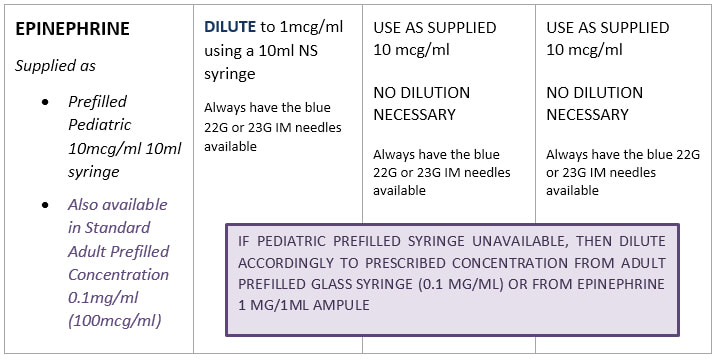
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook

Serial Vs Direct Dilution Time To Apply New Thinking To Ic50 Determination And Dose Response Analysis Drug Discovery World Ddw
Some Iv Medications Are Diluted Unnecessarily In Patient Care Areas Creating Undue Risk Institute For Safe Medication Practices
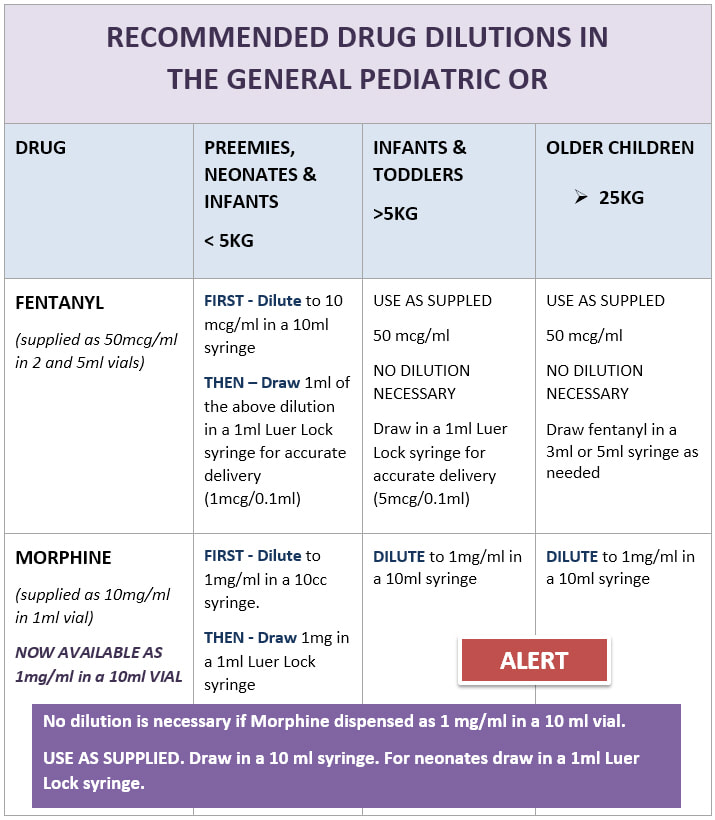
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook

Photo 46 Medication Dilution Chart Posted At The Nursing Station For Download Scientific Diagram
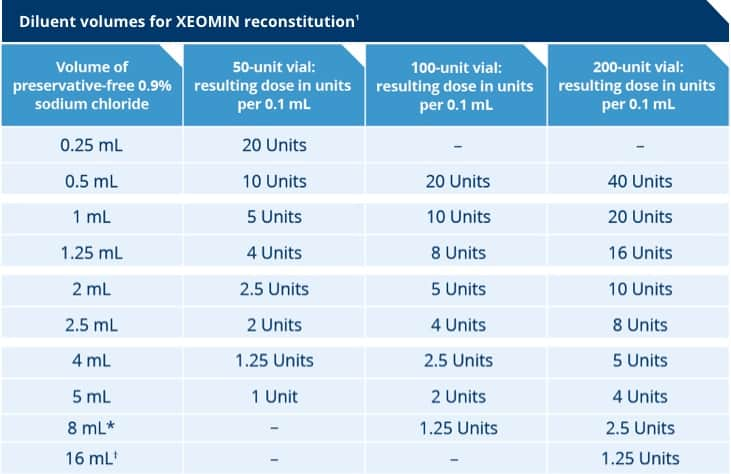
Reconstitution Dilution And Storage Xeomin

Intravenous Iv Preparation And Infusion Guidelines Globalrph
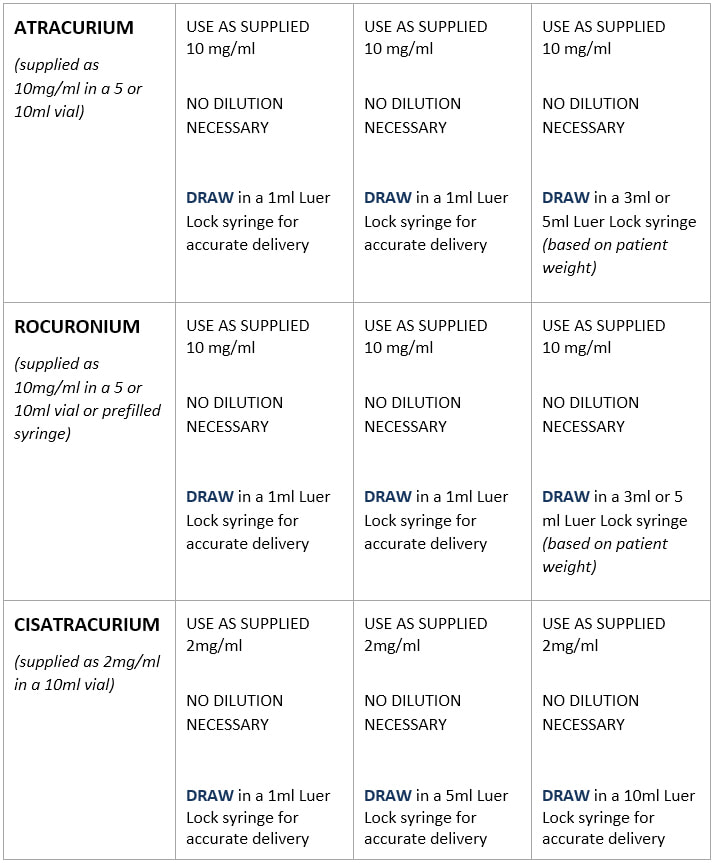
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook

Drug Dilutions Used For Intradermal Skin Testing Download Table
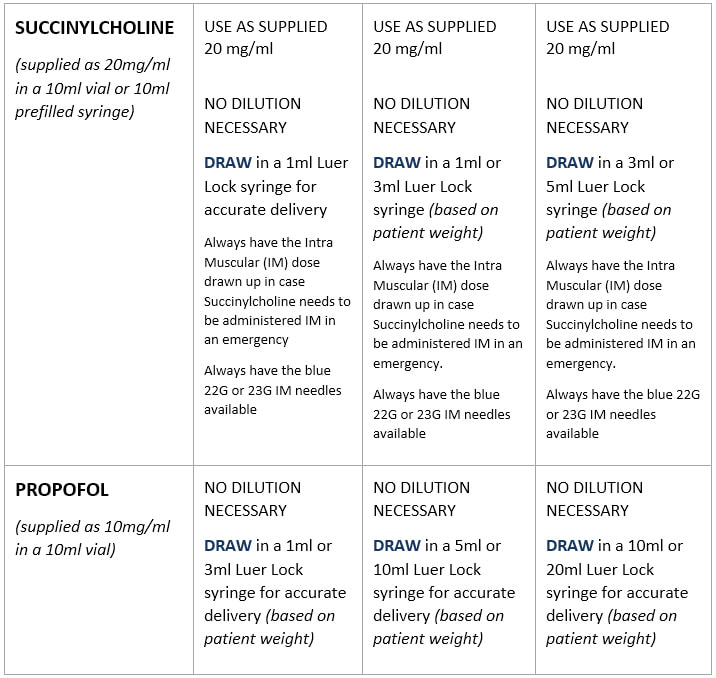
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook
Wall Chart Drugs Given By Iv Push Or Rapid Administration In Adults Institute For Safe Medication Practices





0 Response to "drug dilution guidelines"
Post a Comment